Application Lifecycle Management (ALM) is the heart of any software development or validation. I can take it even further. It must be the heart of any validation or qualification.
In my experience which spans over three (3) decades, 90% of the Life Science companies are glued to MS Word or Excel just like ants to honey! All documents whether it is an URS or FDS or a test script is still written in Word, printed and manually executed. We need to ask what an AI chatbot thinks of this fatal attraction!
Some of the life science companies use a standard document management app which is like driving a square peg in a round hole. They are absolutely not designed to manage a Validation or a Software Lifecycle.
Life science companies need a robust, intuitive ALM platform that can manage project of any type (CSV, SDLC, Equipment, Facilities, Process, etc..). The core elements of any ALM (SUT, Requirements, Specifications, Test Cases, Traceability, Risk Management, Coverage Stats, etc..) must be easy to use out of the box and support integration with other apps. This will enable ALM to be the single source of truth.
These are the drivers at xLM to help our customers manage their life cycle in an agile fashion with real time metrics. We have partnered with xQUAL to rollout ContinuousALM.
ContinuousALM: Key Features
Complete Application Life Cycle Management
ContinuousALM lets you manage requirements, specifications, traceability, test cases (both manual and automated) as well as deviations. You can:
- Structure your releases down to components
- Define your business requirements
- Write your functional and technical specifications
- Manage your projects using Agile/V-model practices
- Design your tests and test case procedures
- Orchestrate your test and test plans
- Plan and execute your test campaigns
- Archive and compare your test results
- Report on coverage, results, progress, quality etc.
- Track your bugs (integrated or third-party)
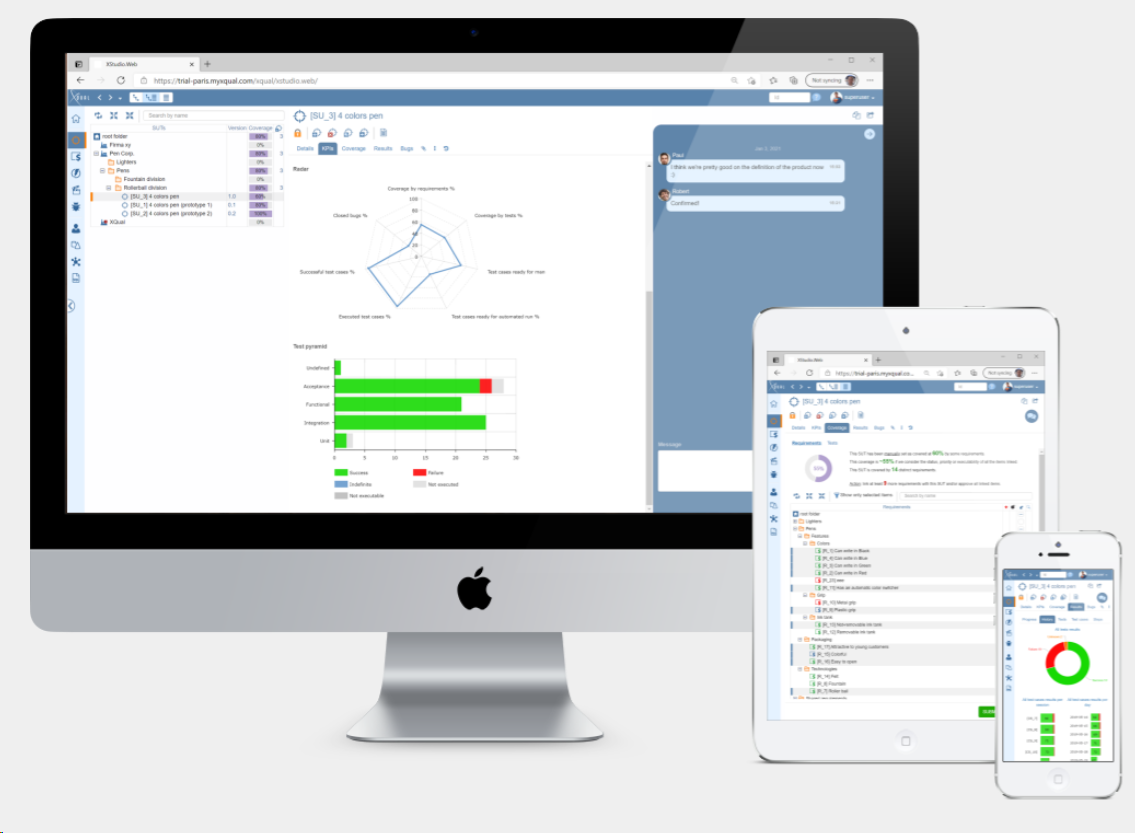
ContinuousALM: A Managed Continuously Validated ALM
System Under Test (SUT) Management
Gain unparalleled insights into your testing activities, ensuring comprehensive coverage and quality metrics. Each SUT, whether it's a hardware device, equipment or a software component, is identified by its name and version, ensuring precision and clarity throughout your testing process.
SUTs are seamlessly organized within a framework of companies, folders, and sub-folders, allowing for systematic organization and management efficiency.
Coverage by Requirements, Specifications, and Tests
With ContinuousALM, you'll have access to comprehensive coverage analysis: Direct coverage by requirements; Indirect coverage by specifications. And let's not forget about indirect coverage by tests, where every aspect of your testing process is factored into to ensure nothing falls through the cracks.
However, what truly sets ContinuousALM apart is the flexibility it offers. Coverage percentages can be manually adjusted based on linked requirements, empowering you to fine-tune coverage levels with precision. And with specification weight determined by priority levels, you can be rest assured that every aspect of your testing process is aligned with your project's goals and priorities.

Requirements and Specifications Management
With our platform, requirements management becomes a breeze, whether you're working directly within Continuous ALM or retrieving requirements from external systems like JIRA, VersionOne, or other third-party systems.
Our requirements tree provides essential information at your fingertips, including the total number of requirements, their distribution across folders, and the status of each requirement—indicated by intuitive icon colors. Moreover, with our coverage column, you'll gain insights into the completeness of requirement coverage, empowering you to make informed decisions. And with customizable coverage indicators accessible via the Coverage drop-down menu, you can tailor your view to focus on specifications or tests, ensuring every aspect of your project is accounted for.
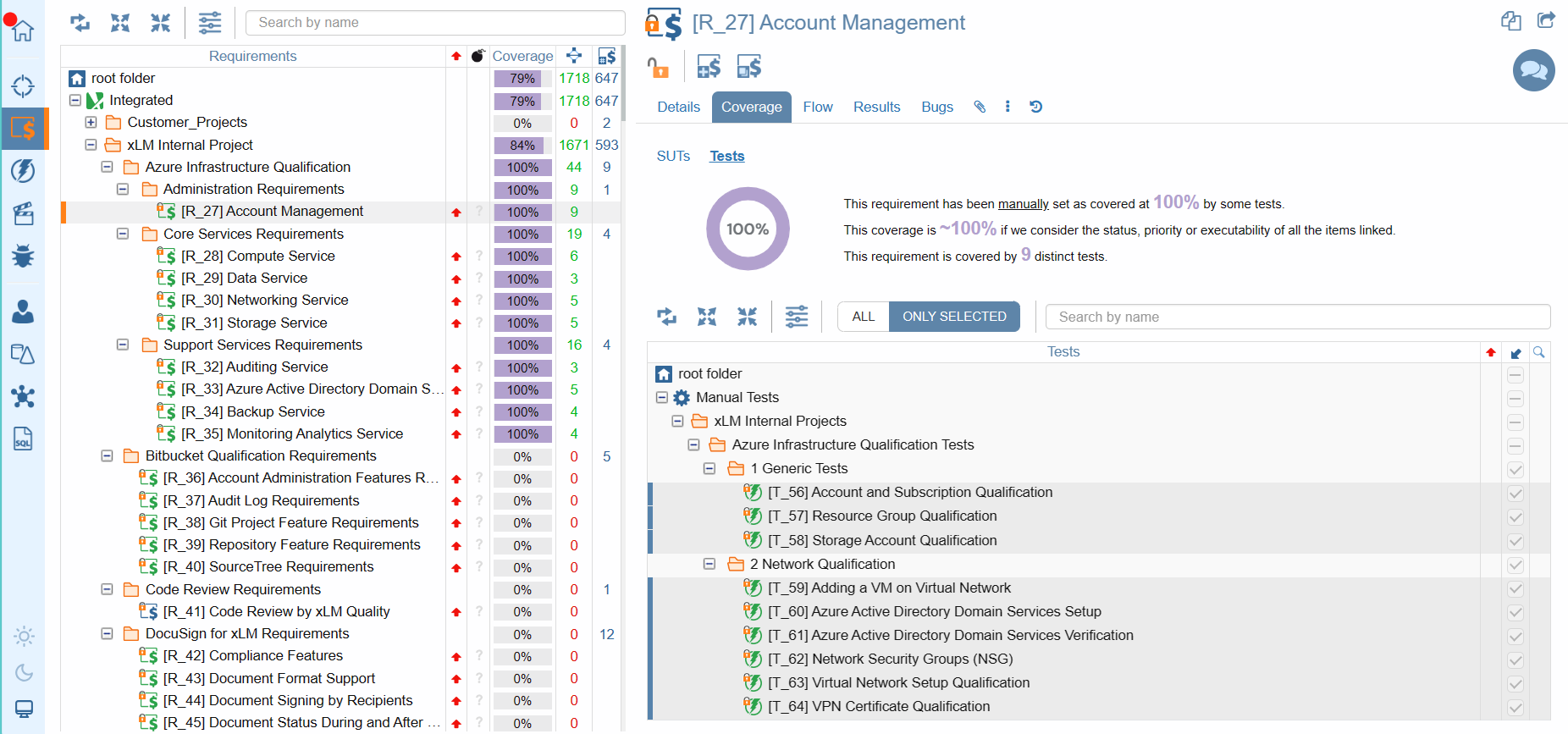
Requirements Coverage Matrix
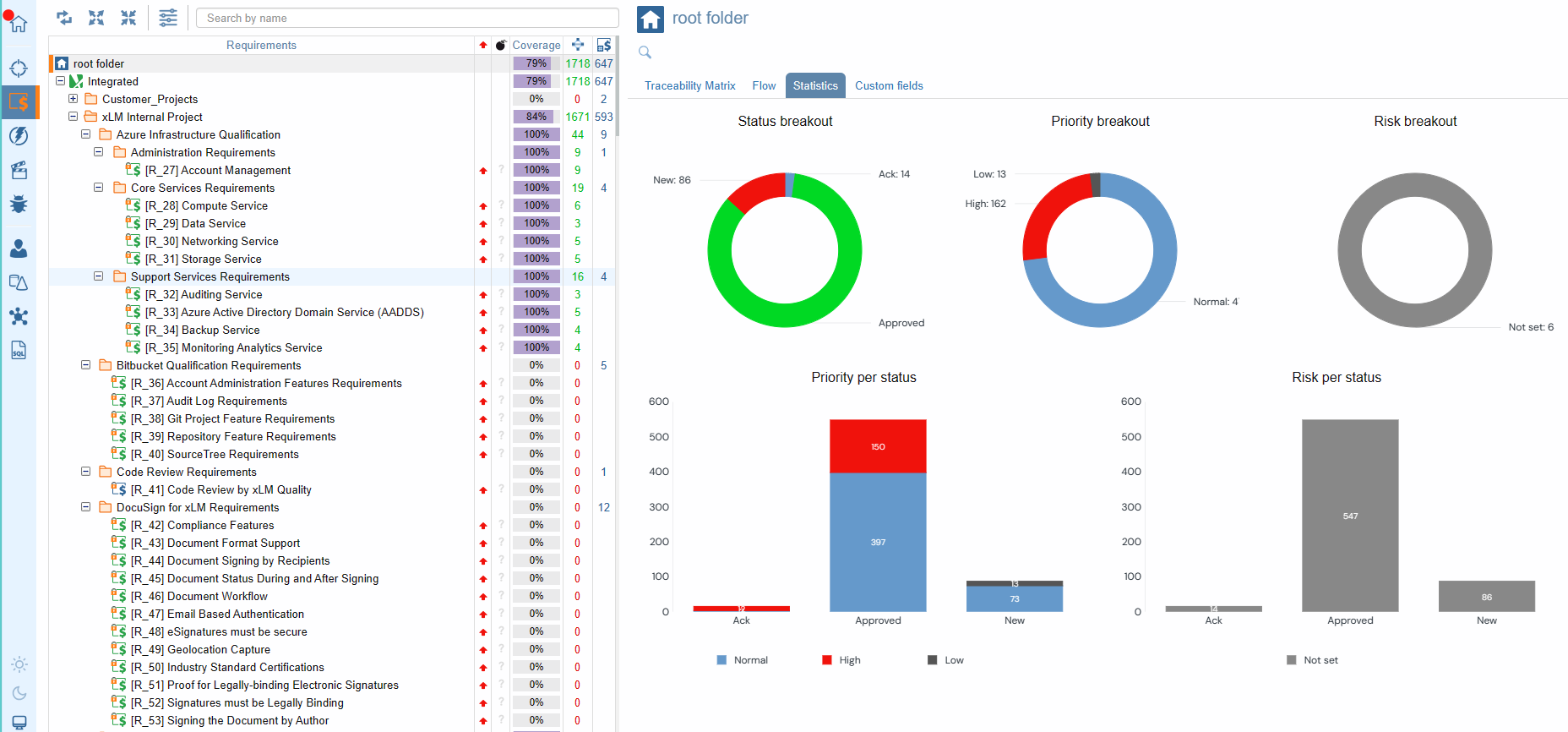
Requirements Statistics
Requirement Risk Management
You can assign a risk level to each requirement. Not only does it streamline reporting processes, but it helps you in selecting and prioritizing tests. Imagine creating a test campaign and having the system automatically select tests based on specific criteria like risk.
The risk is calculated from the failure probability and the impact of a failure on the functionality. In addition, the probability is calculated from the Frequency at which the feature will be used, the Complexity of its implementation and the Maturity of the its components.
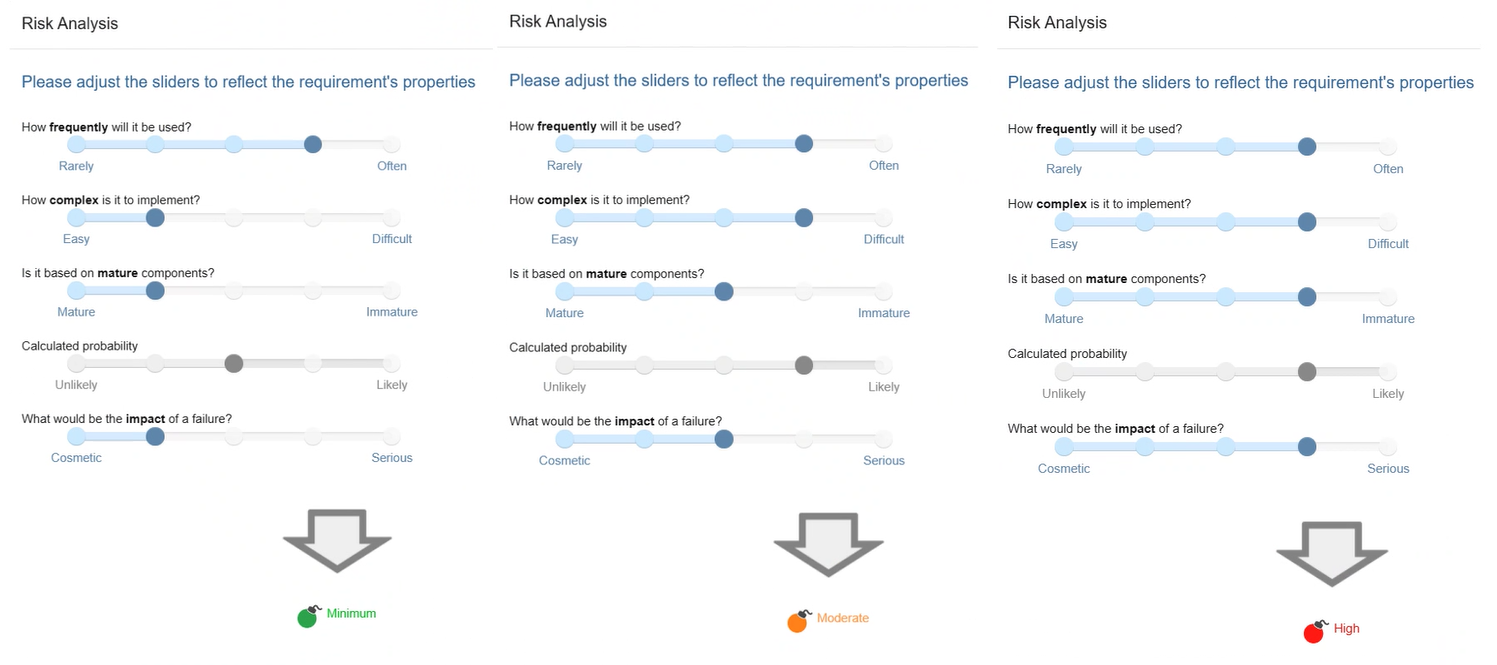
Risk Analysis
Test Management
With ContinuousALM, organization is key to success. Tests are arranged in specific folders following the same structured approach as specifications. However, for software APIs, tests take on a whole new level of precision, with each test meticulously organized per function/method and grouped by function categories. This not only simplifies test suite extension but also paves the way for stress tests, negative tests, and beyond. With ContinuousALM, organization is easy to manage and leverage efficiently.
ContinuousALM's testing module isn't just a tool; it's a powerhouse of features designed to empower teams to ensure comprehensive testing and maintain the highest standards of software quality.
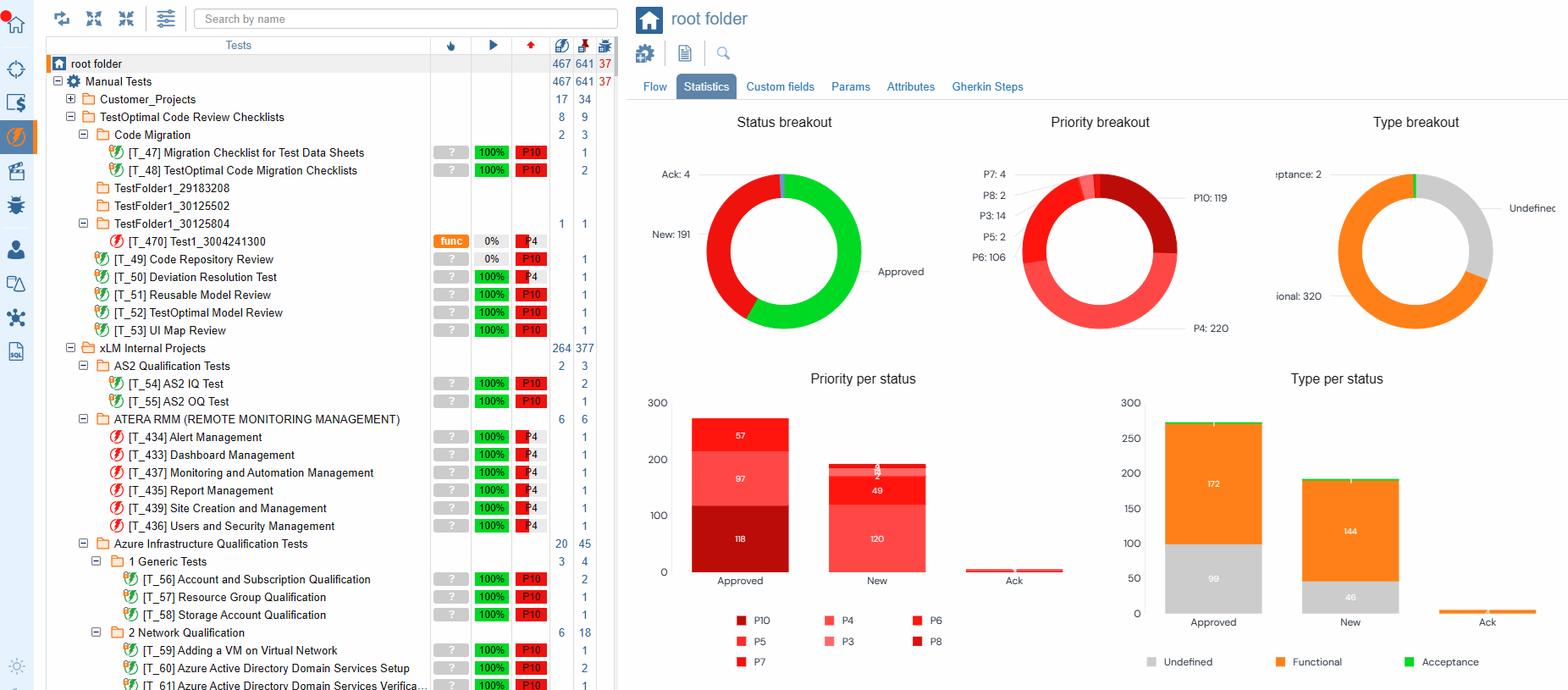
Statistics for Tests and Test Cases
Test Campaign Management
With ContinuousALM's campaign module, organizing, executing, and evaluating test campaigns has never been easier. Our campaigns tree features a coverage column that goes beyond mere estimates, offering insights into the quality of each campaign and session. With quality indicators providing a percentage estimate of test success, you'll have a clear picture of your system under test performance. Moreover, with quality metrics consolidated at the folder/root folder level, you can be rest assured that success is shared across all related tests.
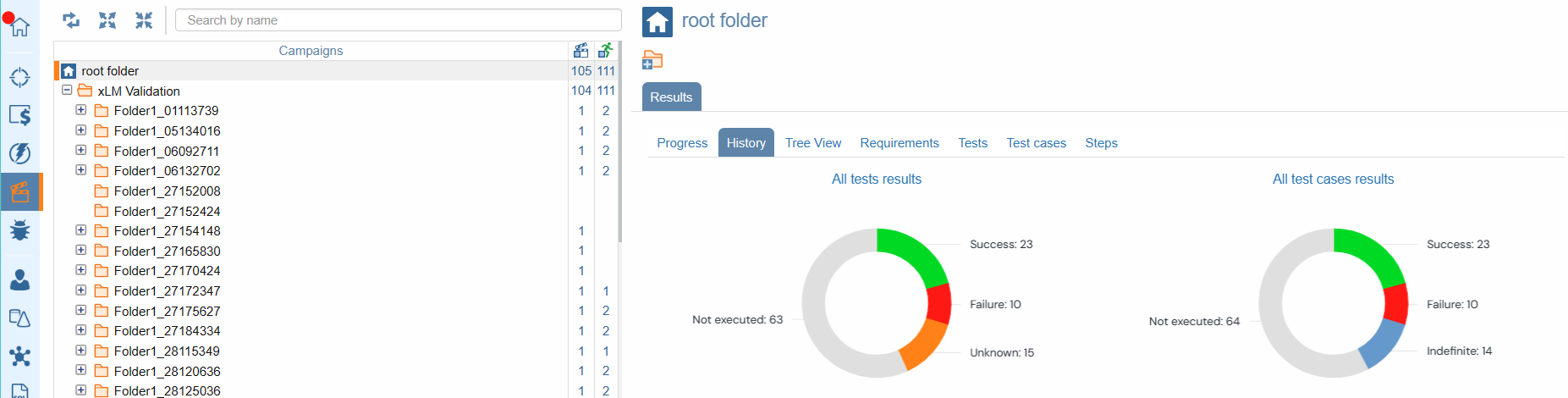
Test Result Campaign Statistics
Defect (Deviation, Discrepancy) Management
With ContinuousALM, every defect finds its home in a centralized repository, ensuring that no bug goes unnoticed. Dive into the defect tree and discover a wealth of essential information, from the total number of defects to their distribution across categories and folders. Moreover, with real-time status updates on each defect—indicated by intuitive icon colors—plus severity and priority ratings for added clarity, you'll have everything you need to tackle bugs head-on.
With ContinuousALM, bug tracking isn't just about identifying issues—it's about taking action. Our platform empowers test operators to conduct thorough report analysis, associating failed test cases with defects to ensure comprehensive tracking and resolution of issues.
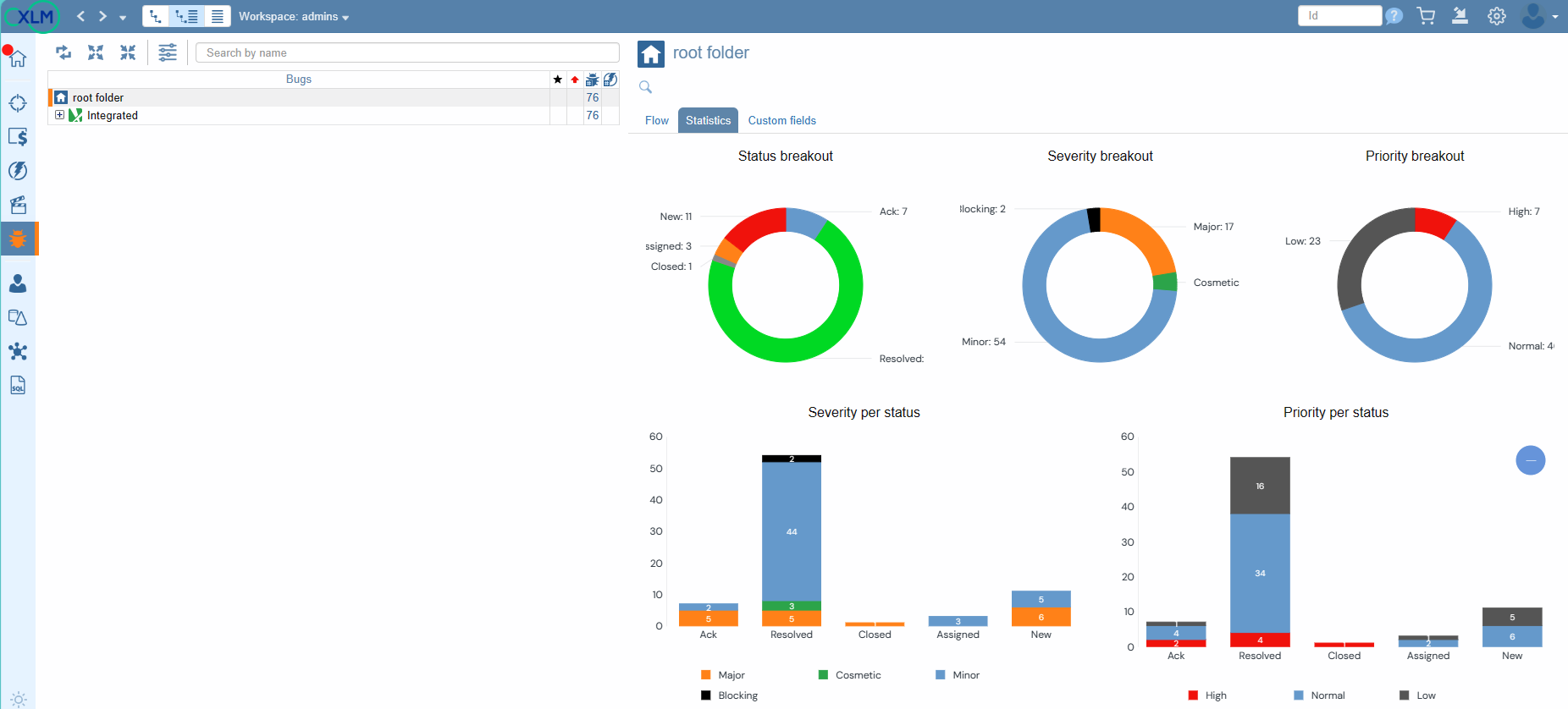
Bugs Statistics
Compliance with Part 11
Whether you're exploring the evolution of a System Under Test (SUT), requirements, tests, campaigns, or defects, ContinuousALM puts the entire history at your fingertips.
Part 11 features:
-
Detailed audit trial at every object level
-
Granular role based security
-
Object level version control
-
Ability for QA to freeze/unfreeze objects
-
Support for e-signatures
-
Bi-directional Traceability
-
Integrated continuous validation at the Product Level and Client Instance Level
-
Delivered as a continuously validated managed service
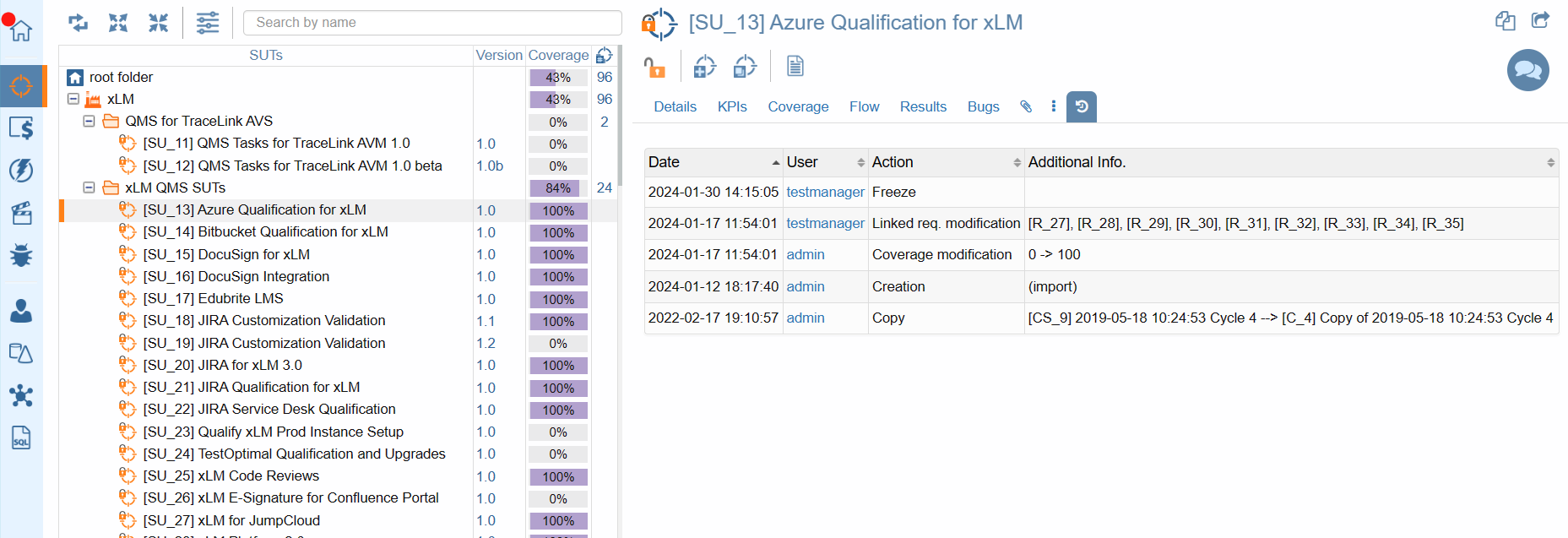
Audit Logs
Traceability, Transformed
ContinuousALM, traceability takes center stage, empowering you to prove the integrity of your lifecycle items with ease. Whether it's ensuring that an item remains unmodified after a certain date or preventing unauthorized changes, our platform has you covered. From SUTs to bugs and everything in between, ContinuousALM's traceability features provide transparency and trust.
Seamlessly trace requirements, specifications, tests, and defects throughout the entire lifecycle. It provides full support for bi-directional traceability
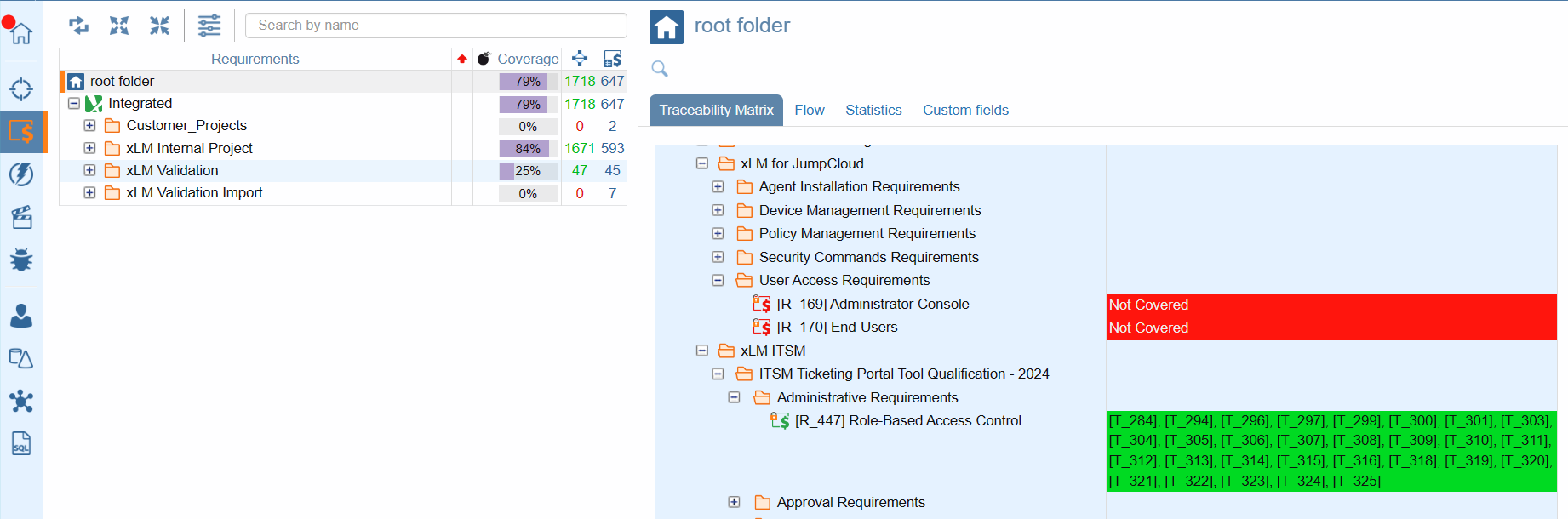
Traceability Matrix
Built-in Support for Part 11 Compliance
From SUTs to tests and beyond, our platform empowers you to freeze items instantly, ensuring their immutability and aligning seamlessly with FDA 21 CFR Part 11 requirements.
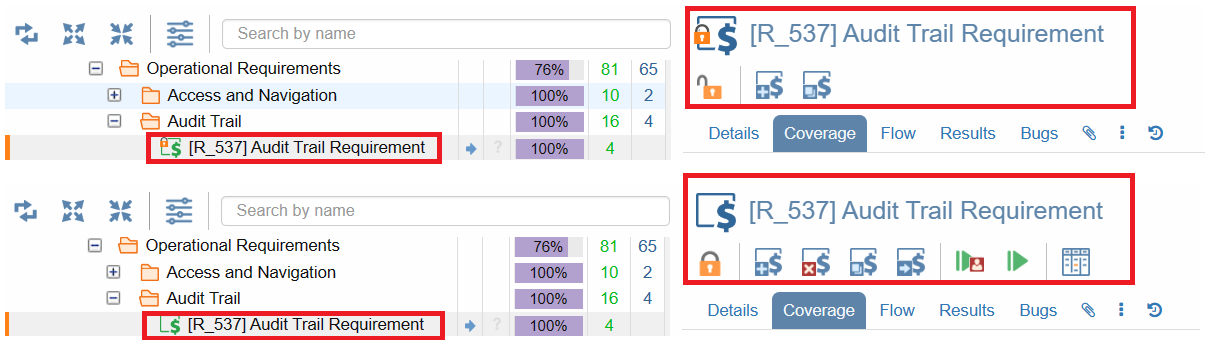
Freezing-Unfreezing
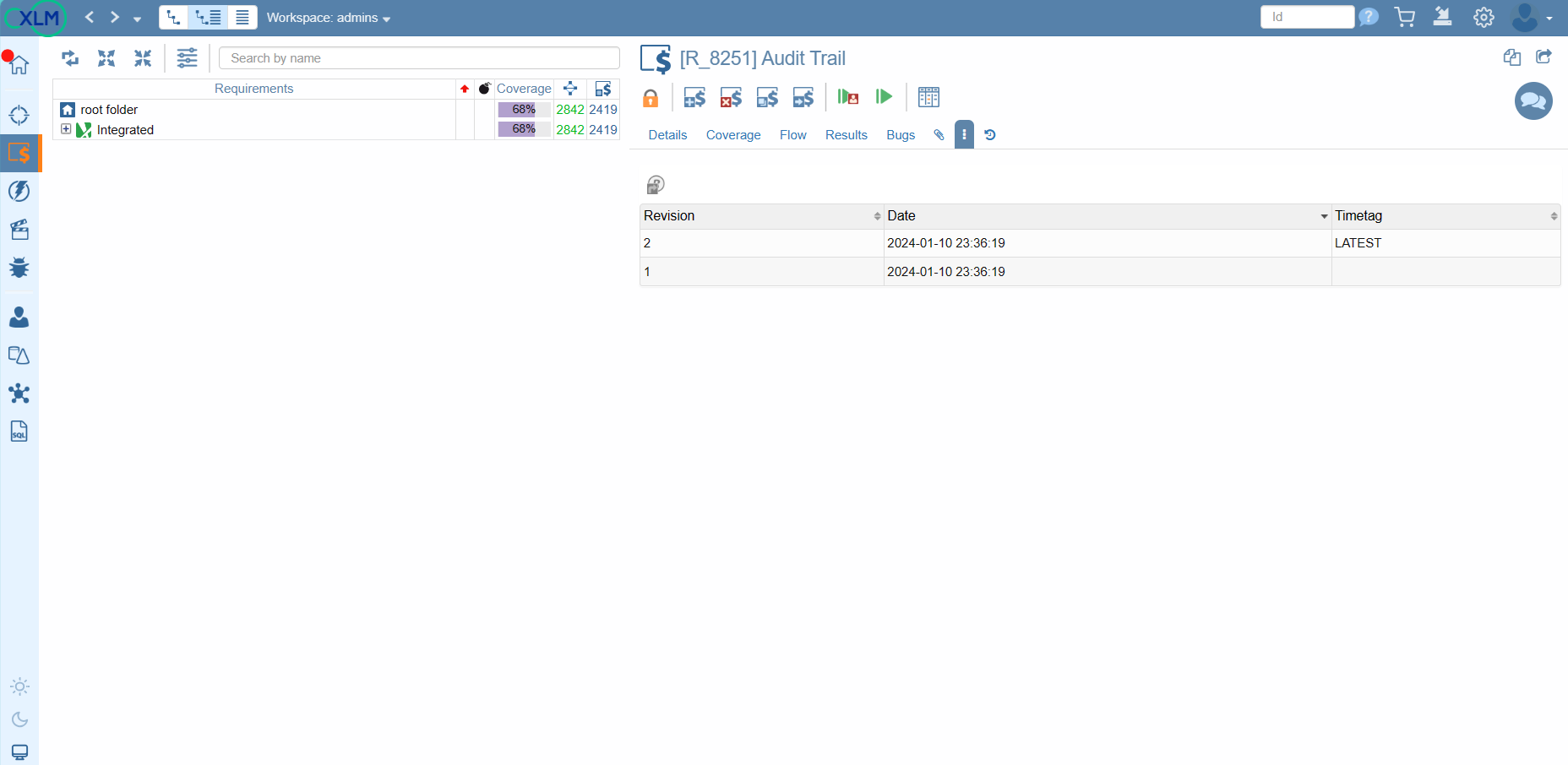
Versioning
Integration Features
Our platform offers flexibility in automation strategies, including an extensive launcher catalog, framework independence, and compatibility with diverse technologies and products. With over 90 launchers at your disposal, executing automated tests has never been easier. Our platform seamlessly integrates with a vast array of automation frameworks and tools, including QTP/UFT, Selenium, JMeter, and many more.
Our tech stack offers a comprehensive set of connectors to integrate with requirements management to bug tracking, automation, and data import capabilities. With dedicated plugins for JIRA, Quality Center (QC), and seamless SQL connections to Mantis, integrating with your favorite tools has never been easier.
Plus, our platform seamlessly integrates with a variety of CI tools, including Bamboo, Jenkins, TeamCity, and more.
Reporting Redefined
From system functionality to test results, compliance status, and risk mitigation strategies, our platform provides comprehensive insights that empower stakeholders to make informed decisions.
Generating User Requirements Specifications (URS), Test Plans, and Traceability Matrices has never been easier. There is built-in support to generate these reports in Word format using your corporate templates.
ContinuousALM provides in real-time the smart progress charts. It covers scope, testability, quality and specifications. You can easily assess your project or product progress.
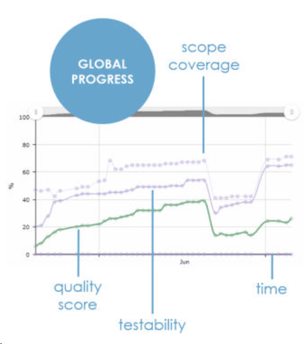
Smart Progress Charts
What's the overall quality of your product, project or system?
ContinuousALM uses complex algorithm taking into account status, priority, executability, local coverage of each component in the traceability matrix, latest results on all the elements to calculate a unique Quality Score
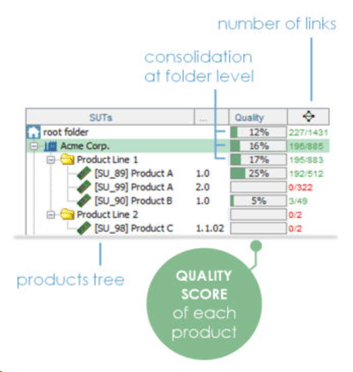
Quality Scoring
Are you improving your quality?
ContinuousALM presents the tests distribution (also called tests pyramid) for each product or project. By visualizing the spread in test types, you can immediately assess if your development cycle supports your quality objectives. You also see how your team is embracing proven practices such as Test-Driven Development (TDD), Behavorial-Driven Development (BDD), etc.
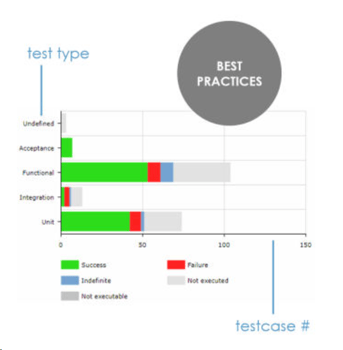
Tests Pyramid
KPIs and Data Visualization
-
One-click progression assessment on requirements and specifications completion
-
One-click testability and quality assessment
-
Radar-based at-a-glance status assessment before system release
-
Quality-driven assessments using the test pyramid
-
Dimension-based and time-based data analysis diagrams for any object
-
Export reports in xml, html, csv (Excel), docx (Word) and pdf formats
-
System, Requirements, Specification coverage/results
-
History of all test executions
-
Campaign coverage/results/trends/progress
-
Campaign results comparison (per configuration/operator/agent)
-
Defect (Deviation) metrics per status/per user
-
Defect submission rate vs resolution rate trend
-
Graph of dependencies between tests
-
Tracking of test readiness for manual/automated execution
-
Tracking of bugs status (submission rate vs resolution rate)
-
Tracking of scrum projects (velocity graph automatically generated)
-
Generation of coverage/results metrics on any sub-domains of requirements, specifications and tests
-
Automatic generation of the traceability matrix
Software Quality Built-in
-
Support for automated and manual tests execution
-
Dedicated exploratory testing module based on Session-Based Test Management (supports FDA CSA)
-
Test case parameterization (including an implementation of the pairwise algorithm)
-
Real-time execution graph stored
-
Dependencies between tests
-
Automatic scaffolding of tests from Requirements or specifications
-
Support for Bidirectional Traceability including traceability to Campaign Sessions (Execution Runs)
ContinuousALM - Delivered as a Managed Service
In every service we offer, the software app is continuously qualified. Also the customer's instance is continuously validated. In each run, 100% regression is performed.

Continuous Validation Features
AI Features (Coming Soon)
-
An AI agent will automatically generate test cases from exploratory tests.
-
An AI algorithm will validate the traceability to ensure accuracy and coverage.
-
An AI agent will determine the tests to be run based on the changes.
-
and more....
Conclusion
If you are looking at managing your software, validation or qualification life cycles, then ContinuousALM delivers a rich feature set that can get you going out of the box. Since it is delivered as a Managed Service, you can be up and running the same day. You don't have to worry about any validation document or executing any test protocol. It is delivered as an app that is continously validated.
When you take the rich feature set, real-time KPIs, bi-directional traceability, useful reports, built-in Part 11 compliance and its ability to integrate with any enterprise app, there is only one option for a robust ALM: ContinuousALM.
Latest AI News
- ChatGPT can now remember!
- It’s for Real: Generative AI Takes Hold in Insurance Distribution.
- GitHub has launched a new AI-powered tool in beta, designed to automatically detect and fix security vulnerabilities in code.
- Imagine diagnosing eye diseases 100 times faster - it's now reality. AI has just revolutionized retinal imaging.
- Is AI the New Frontier in Continuous Validation in Life Sciences?
What questions do you have about artificial intelligence in Life sciences? No question is too big or too small. Submit your questions about AI via this survey here.
ContinuousALM is built-on xQUAL. xLM, LLC incorportes best practices, automation, reporting tools and configuration settings to make it GxP / 21 Part 11 compliant. xLM LLC delivers ContinuousALM as a continuously validated app to its customers.
ContinuousALM FAQs
|
Question |
Answer |
|---|---|
|
1. What is ContinuousALM and what are its key features? |
ContinuousALM is a comprehensive Application Lifecycle Management (ALM) platform designed specifically for the life sciences industry. It moves beyond traditional document-centric approaches, providing a centralized hub for managing all aspects of software development and validation. Key features include:
|
|
2. How does ContinuousALM address the limitations of traditional document-centric approaches in the life sciences? |
Traditional reliance on MS Word and Excel for managing validation and software lifecycles presents several challenges:
ContinuousALM addresses these limitations by:
|
|
3. What is SUT Management in ContinuousALM and why is it important? |
SUT Management in ContinuousALM allows you to effectively organize and manage all your Systems Under Test (SUTs). Each SUT, whether it's a hardware device, a piece of equipment, or a software component, is clearly identified by its name and version. This is crucial because it:
|
|
4. How does ContinuousALM handle Risk Management? |
ContinuousALM features a dedicated risk management module that allows users to assign a risk level to each requirement. This risk level is calculated based on the probability of failure and the potential impact of that failure on the functionality. Risk management is essential because it:
|
|
5. What are the benefits of using ContinuousALM for Test Management? |
ContinuousALM's Test Management module provides several advantages:
|
|
6. How does ContinuousALM ensure compliance with 21 CFR Part 11? |
ContinuousALM is designed with built-in features to meet the stringent requirements of FDA 21 CFR Part 11. Key compliance features include:
|
|
7. How does ContinuousALM’s reporting functionality benefit life sciences companies? |
ContinuousALM offers advanced reporting capabilities that provide valuable insights for decision-making and compliance:
|
|
8. What future innovations are planned for ContinuousALM, particularly in the area of Artificial Intelligence (AI)?
|
ContinuousALM is committed to ongoing innovation, and AI is playing an increasingly significant role. Planned AI features include:
These AI features are designed to further enhance ContinuousALM's capabilities, automate manual processes, and empower life sciences companies to achieve higher levels of efficiency, quality, and compliance. |
|
9. How can I get started with ContinuousALM?
|
If you're ready to experience a streamlined, efficient, and compliant application lifecycle management solution, contact xLM to learn more about ContinuousALM and how it can benefit your organization. |


COMMENTS